Promoting Brain State Transformation from Early Mild Cognitive Impairment to Health through Virtual External Stimulation
Promoting Brain State Transformation from Early Mild Cognitive Impairment to Health through Virtual External Stimulation
Alzheimer’s disease is an incurable neurodegenerative disease that often starts with mild cognitive impairment (MCI) and progresses to AD. Research has focused on early MCI as a potential target for therapeutic interventions to delay disease progression. Neurostimulation techniques like tDCS and rTMS have shown promise in improving cognitive function in patients with AD and EMCI. However, large-scale experimental studies on these techniques are limited due to experimental and ethical constraints. Computational brain models based on brain activity have been used to understand brain dynamics and guide neurostimulation. Neuroimaging techniques have also been instrumental in studying brain states and identifying functional connectivity changes in diseases like MCI. Dynamic functional connectivity studies, such as LEiDA analysis, have provided valuable insights into various neurological disorders including Alzheimer’s disease.
The specific brain areas can play a crucial role in directing the brain to a desired state and impacting cognitive performance. Previous research has shown that nodes with high average controllability can transfer the brain state to a nearby state, while nodes with high modal controllability can drive the brain to a distant state. However, these controllability indicators do not provide information on the number of targets needed for full control. To address this, exact controllability based on algebraic maximum multiplicity can determine the minimum set of drivers required for complete control in any network.
Recently, researchers from University of Science and Technology Beijing (Weiping Wang, Weiwei Wang, Haiyan Zhao, Zhen Wang5, Xiong Luo and Jipeng Ouyang) used LEiDA to cluster dynamic functional connections based on fMRI data and constructed a brain model combining structural connectivity from DTI data. By identifying the minimum set of driving nodes using exact controllability, they aimed to achieve complete control of effective connections in patients with EMCI. Finally, they applied external interference to these driving nodes to determine the optimal brain regions that can promote the transition from EMCI to a healthy state.
Their computational model
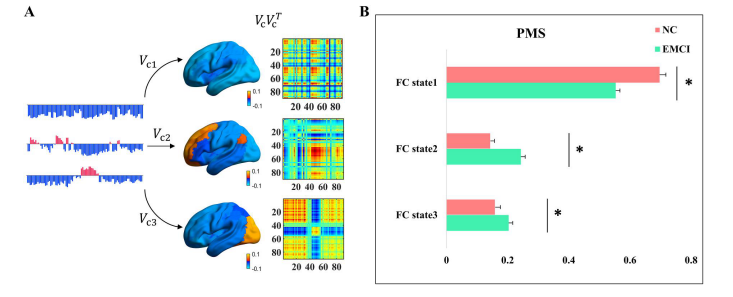
A whole-brain computational model consists of 90 nodes representing brain regions grouped by the AAL template. The model describes each brain region’s local dynamics using a type of supercritical Hopf bifurcation known as the Landau-Stuart oscillator. By simulating the BOLD signals at the whole-brain level, the model allows for the investigation of how activation of different nodes affects the brain network. Mathematically, the model is represented as a coupled kinetic equation for each brain region, where the global dynamics are coupled by the structural connectivity. The parameters of the model, such as the bifurcation parameter and global coupling parameter, are optimized to fit the empirical data from patients with early mild cognitive impairment (EMCI). The optimal operating point of the system is determined by calculating the Kullback-Leibler distance between the simulated and empirical probabilities of different substates. The model was validated by comparing the simulated BOLD signals with the empirical data using the leading eigenvector dynamics analysis (LEiDA) to derive the probabilistic metastable substate (PMS) space.
They found that three substates could significantly distinguish between the two groups, with patients having a lower chance of being in substate 1 and a higher chance of being in substate 2. Patients with EMCI also exhibited enhanced synchronization within the visual network. To further investigate the brain states of patients with EMCI, the authors constructed a kinetic model consisting of 90 brain regions coupled by the structure matrix obtained from DTI data. They optimized the model parameters by calculating the shortest KL distance between the simulated and empirical probabilities of the PMS space. The bifurcation parameter and global coupling parameter were adjusted to find the optimal fit, resulting in three groups of simulations with different g values. The authors achieved a good fitting effect of the brain states of patients with EMCI using the Hopf calculation model.
How they determined the stimulation target
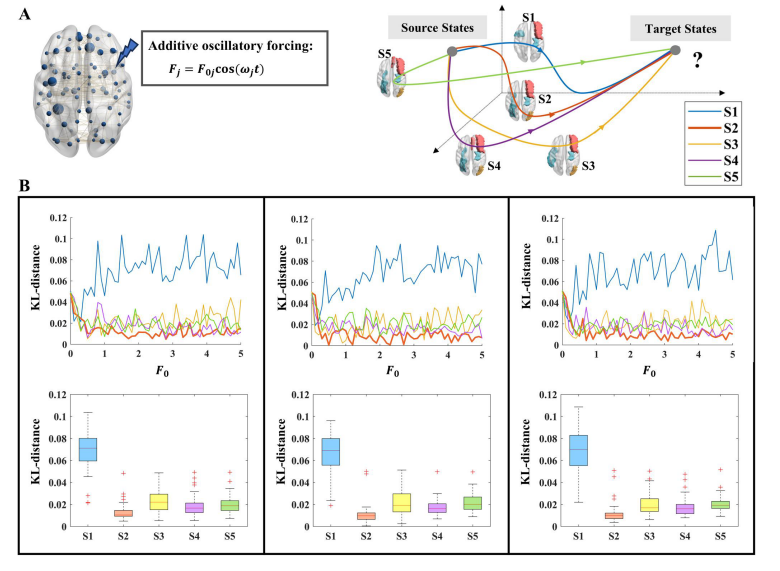
The concept of brain controllability is used to measure how easily the brain can transition from one state to another. In this study, the researchers utilized exact controllability in linear network control to examine the ability of brain regions to influence other regions. They used a simplified linear model of network dynamics to describe the neurodynamics of fMRI measurements. By applying the Popov-Belevitch-Hautus (PBH) rank criterion, they determined the minimum number of driving nodes required for complete control of the brain network. The controllability problem involved finding the position of the driving nodes that satisfied the rank criterion. To force the transition from EMCI to health, the researchers used the whole-brain model and applied external stimuli to the minimum set of driving nodes. The stimuli were presented as periodic forcing terms. By perturbing the selected nodes, they explored their ability to drive the transition from EMCI to health, as measured by the KL distance between the post-stimulation brain state and the healthy brain state. This process was repeated for different amplitudes of disturbance.
To search for stimulation targets in patients with early mild cognitive impairment (EMCI), the researchers utilized exact controllability in brain networks. They first identified the minimum number of driving nodes that could achieve complete control of effective connectivity. Their locations were explored as potential stimulation targets. The study focused on 42 brain regions related to cognition, located in the frontal, parietal, occipital, and temporal lobes. The effective connection matrix was calculated to estimate the direction and strength of information transmission between these regions. The researchers then sparsely binarized the effective connectivity matrix to preserve stronger connections. The minimum number of driving nodes was identified as 3, 4, or 5, depending on the criteria used. Five sets of stimulation targets were selected, which included the hippocampus, medial frontal gyrus, suboccipital gyrus, and fusiform gyrus. The researchers then applied external circulatory stimuli to these stimulation targets in the whole-brain model. The effectiveness of each stimulation target set in transitioning from EMCI to a healthier state was compared using the Kullback-Leibler distance. The second stimulation target set performed the best in promoting the transition.
The meaning of their study
In this study, the LEiDA method was used to analyze the evolution of brain substates over time in patients with early mild cognitive impairment (EMCI). The dominant connectivity model was applied to study the abnormal dynamics of the brain in these patients and to identify state dynamics characteristics associated with cognitive changes. Exact controllability was then used to determine the set of driving nodes that could enable full controllability of the brain network and facilitate the transition from EMCI to a healthy state using a Hopf bifurcation model. The findings suggest that the underlying mechanisms of cognitive decline in EMCI patients can be explored using the LEiDA method. The Hopf bifurcation model was successfully used to depict brain states and optimize external stimulation strategies to facilitate the transition from EMCI to a healthy state. The study also identified key brain regions, such as the hippocampus, medial frontal gyrus, suboccipital gyrus, and fusiform gyrus, which were optimal targets for stimulation to achieve state transition in EMCI patients. However, further investigation is needed to validate the methods used and overcome certain limitations of the study, such as the selection of preprocessing techniques and the stimulation intensity. Overall, this study provides insights into the dynamic functional connections in the brain and potential strategies for intervention in EMCI patients.